Take Action!
Concerned? In less than a minute, send a message to your Health Minister below:
News Ticker
What you need to know about forced switching
BIOSIMILARS 101

FORCED SWITCHING
Which biologic or biosimilar to prescribe is a decision that should be made by physicians, in consultation with their patients. But in some jurisdictions, governments are forcing patients to switch from their physician chosen biologic (or biosimilar) to a government-chosen product – not for medical reasons, but so that governments can save money.
A BETTER WAY TO FIND SAVINGS
Containing health care costs is important. There’s a better way to reduce costs than forced switching. In the vast majority of countries, the payer continues to reimburse multiple products—biosimilars and biologics alike. In many countries, the physician is encouraged to choose the lowest priced product for new patients.

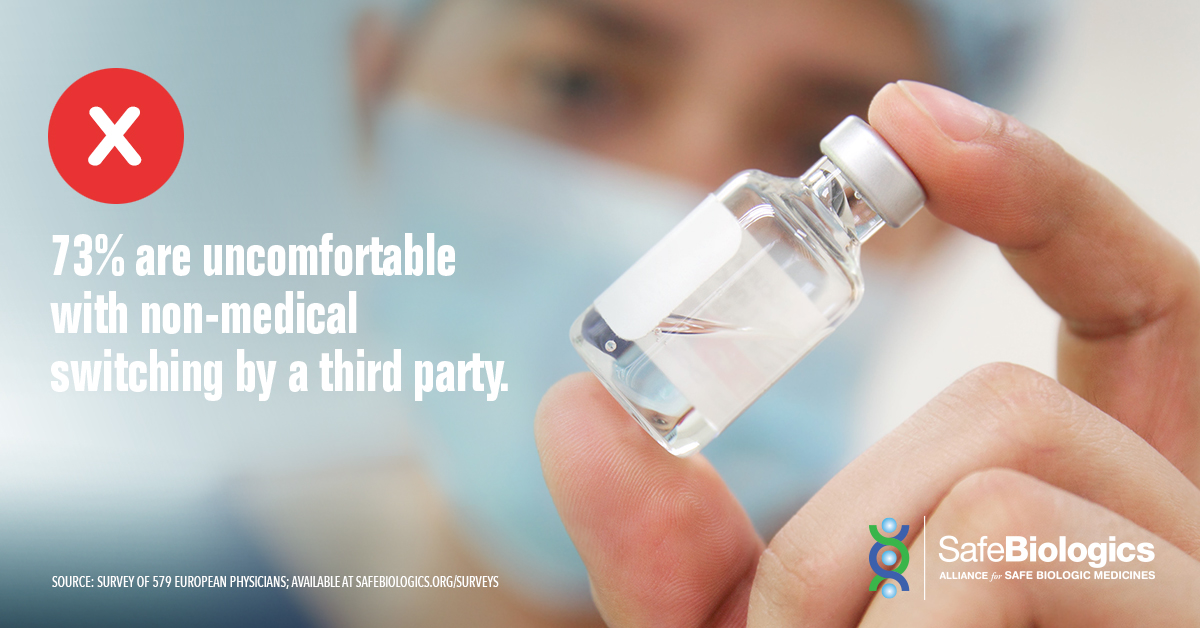
CANADA’S PHYSICIANS, PATIENTS OPPOSE FORCED SWITCHING
- 83% considered it “very important” or “critical” that prescribing physicians decide the most suitable biologic for their patients.

FORCED SWITCHING
Which biologic or biosimilar to prescribe is a decision that should be made by physicians, in consultation with their patients. But in some jurisdictions, governments are forcing patients to switch from their physician chosen biologic (or biosimilar) to a government-chosen product – not for medical reasons, but so that governments can save money. Biosimilars will always have inherent differences from the originator product and from other biosimilars in their product class. While safe and effective products in their own right, due to these differences, most European countries do not allow “automatic substitution”—or forced switching—with biosimilars. Canadian physician societies and patient advocacy organizations have raised concerns with forced switching.

CANADA’S PHYSICIANS, PATIENTS OPPOSE FORCED SWITCHING
“Non-medical switching in patients being treated with a reference biologic is generally not accepted by learned societies and the consulted clinicians.”
– “Safety of switching biologics and their interchangeability”, INESS Report (Quebec), May 2020
In a 2017 survey of 403 Canadian physicians:
- 83% considered it “very important” or “critical” that prescribing physicians decide the most suitable biologic for their patients.
- 64% were uncomfortable with a third party switching a patient’s biologic medicine for nonmedical (e.g. cost) reasons.
Physicians know what is best for patients—that’s why they oppose governments forcing them to choose a medicine, based purely on cost considerations.
Video: Canadian Physicians Oppose Non-Medical Switching
Read the Joint statement from the Canadian Association of Gastorenterology and Crohn’s and Colitis Canada opposing non-medical switching of IBD patients.

A BETTER WAY TO FIND SAVINGS
Containing health care costs is important. There’s a better way to reduce costs than forced switching. In the vast majority of countries, the payer continues to reimburse multiple products—biosimilars and biologics alike. In many countries, the physician is encouraged to choose the lowest priced product for new patients. The result is a competitive market that drives down costs across the board for the long-term—a better option then the short-term savings of forced switching. Cost savings in Europe were accomplished not by forcing patients to switch to a single government-chosen biologic, but by preserving physician and patient choice.
Shareable Graphics
Share this with your friends on social media by clicking below.
Resources
FACT SHEET
Read the INESSS report on automatic substitution
Open Letter to Ontario Premier and Deputy Premier/Minister of Health
Joint Statement from the Canadian Association of Gastroenterology and Crohn’s and Colitis Canada
European Physician Perspectives on Biosimilars
Video: Canadian Physicians Oppose Non-Medical Switching
The Argument Against a Biosimilar Switch Policy for Infliximab in Patients with Inflammatory Bowel Disease Living in Alberta
Joint Statement from the Canadian Association of Gastroenterology and Crohn’s and Colitis Canada
FACT SHEET
Read the INESSS report on automatic substitution
Read the GaBI EU markets paper and learn about Europe’s biosimilars success
Joint Statement from the Canadian Association of Gastroenterology and Crohn’s and Colitis Canada
What are Biosimilars?
What Are Biologics?
Biotech medicine offers the best hope for some of the most devastating medical conditions, such as Alzheimer’s and Parkinson’s disease, for which there are currently no effective treatments. These drugs are also the fastest-growing segment of the pharmaceutical market and are expected to account for half of the new drugs approved by 2015.
Why Is Patient Safety A Concern?
In order to make informed choices, patients should understand the complexity of these drugs and the difference between oral or injected generics versus biosimilars and talk with their doctors about the best course of treatment.

Mission Statement
It is the mission of the Alliance to serve as an authoritative resource center for the public, medical community, and regulators worldwide as biosimilars policies are developed and implemented.
Who We Are
The Alliance for Safe Biologic Medicines is an organization composed of diverse healthcare groups and individuals—from patients to physicians, biotechnology companies that develop innovative and biosimilar medicines and others who are working together to ensure patient safety is at the forefront of the biosimilars policy discussion. Our membership list and steering committee reflect the wide range of voices within the healthcare community. Our coalition ultimately strives to position itself to be an authoritative resource center of information for the healthcare and policy communities.